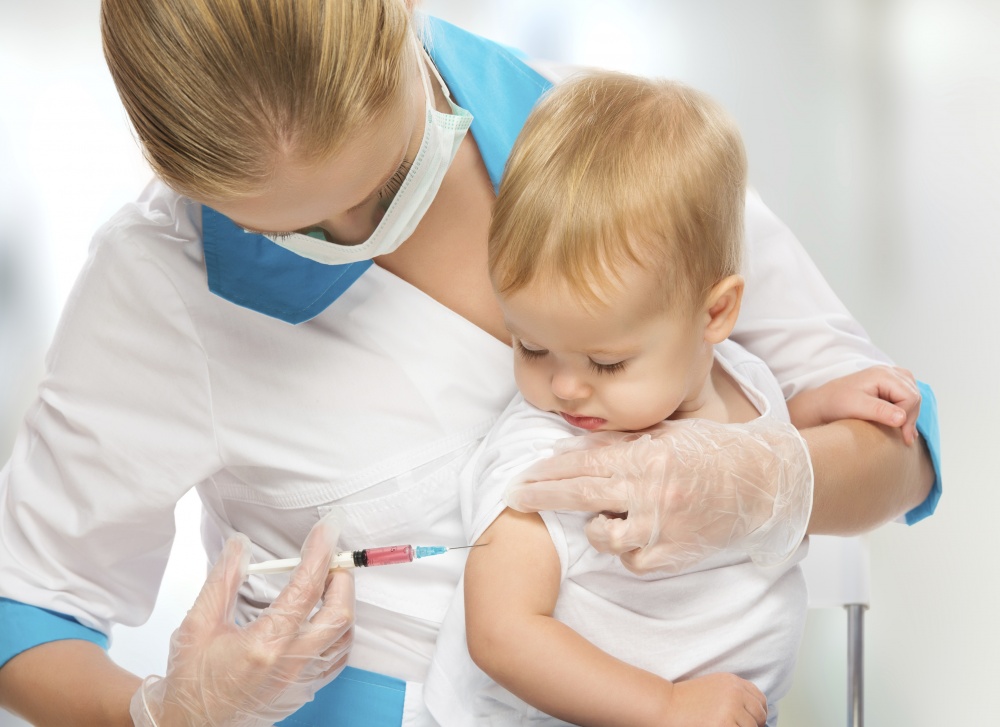
В Китае разгорелся скандал с некачественной вакциной. Завозят ли ее в Кыргызстан?
В Китае обнаружили непригодные для использования вакцины: крупнейший производитель нарушил стандарты по их изготовлению. Об этом сообщают СМИ.
Отмечено, что компания "Чангчун Чангшенг" изготовила не менее 250 тысяч вакцин без соблюдения надлежащих стандартов. Китайцы жалуются на то, что коррупция и злоупотребления в фармацевтической отрасли страны ставят под угрозу жизни простых людей.
Скандал привел к массовым критическим сообщениям в интернете в адрес китайского правительства и пошатнул доверие к нему. Там опасаются, что родители массово начнут отказываться вакцинировать своих детей, хотя это и предусмотрено законом.
Может ли некачественная вакцина повредить кыргызстанским детям?
Как прокомментировала Kaktus.media директор Республиканского центра иммунопрофилактики Гульбара Ишенапысова, в Кыргызстан из Китая вакцины не завозятся. "Мы получаем вакцины из Бельгии, Кореи, Японии, Индии. Только от тех производителей, продукт которых преквалифицирован Всемирной организацией здравоохранения. Это означает, что вакцина безопасна и соответствует международным нормам качества. Кыргызстан заключил меморандум с Детским фондом ООН (ЮНИСЕФ). Закупка вакцин производится только через отдел поставок международной организации", - подчеркнула она.
По словам Ишенапысовой, к примеру, российскую вакцину в Кыргызстан не завозят, так как у нее нет преквалификации ВОЗ.